About MYQORZO™
How MYQORZO works
Mechanism of action
MYQORZO targets the underlying pathophysiology of oHCM1
oHCM CARDIAC SARCOMERE2
In oHCM, there is a change in sarcomeric proteins resulting in excess cardiac contractility3
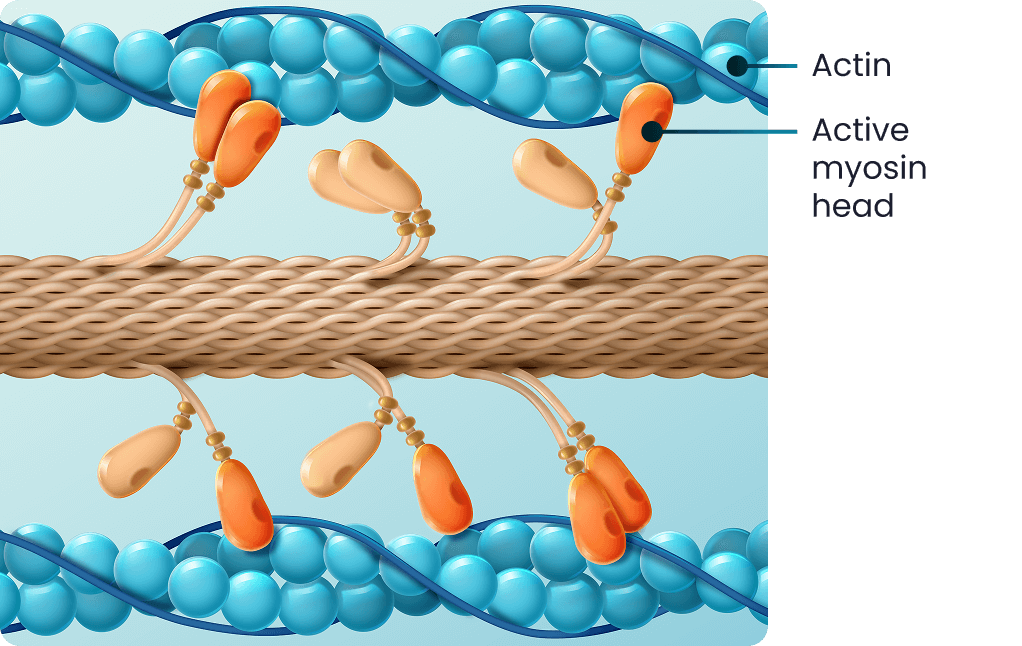
- These changes result in increased actin and myosin cross-bridging in each cardiac cycle3
- This leads to cardiac hypercontractility, impaired cardiac relaxation, and increased energy consumption3

oHCM CARDIAC SARCOMERE WITH MYQORZO1,4
MYQORZO reduces cardiac hypercontractility1
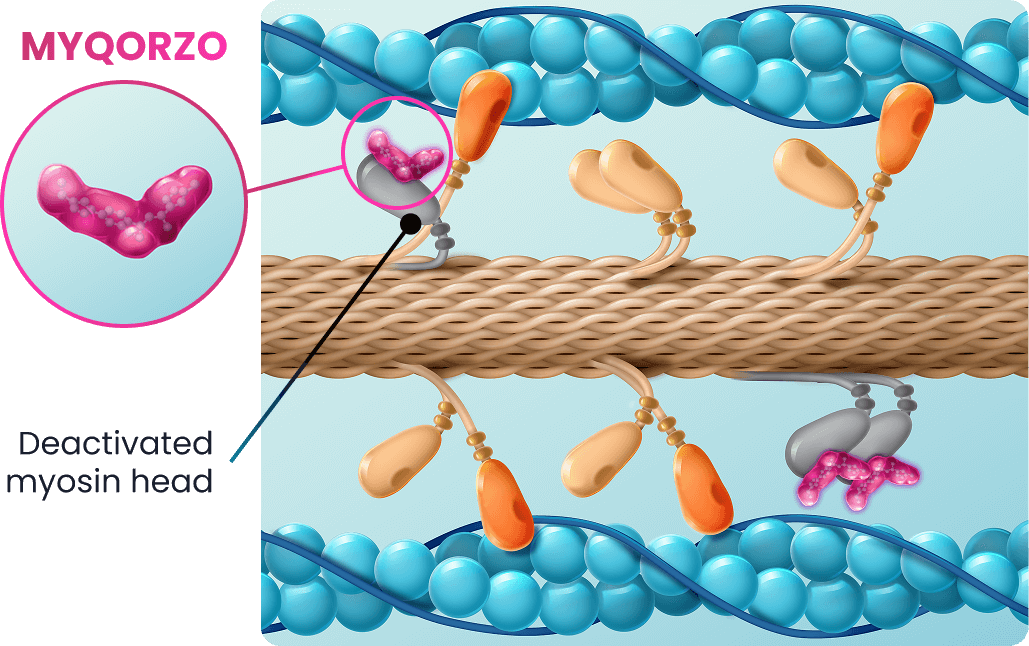
- Binds and inhibits cardiac myosin at a distinct binding site3
- Reduces excessive myosin and actin cross-bridging resulting in3:
- Decreased cardiac hypercontractility and reduced LVOT obstruction1
Clinical Pharmacology
MYQORZO was engineered for optimal pharmacology with quick onset of action and rapid reversibility1,3,5
Low peak-to-trough variability helps mitigate the risk of overexposure or underexposure
Wide therapeutic window expands the safety margin
A half-life of 80 hours allows for rapid reversibility within 24 to 48 hours and the ability to reach steady state at ≈2 weeks (17 days)
Primarily metabolized by CYP2C9, to a lesser extent by CYP3A, CYP2D6, and CYP2C19
CYP=cytochrome; LVOT=left ventricular outflow tract; oHCM=obstructive hypertrophic cardiomyopathy.
References: 1. MYQORZO (aficamten). Prescribing information. Cytokinetics; 2025. 2. Garfinkel AC, Seidman JG, Seidman CE. Genetic pathogenesis of hypertrophic and dilated cardiomyopathy. Heart Fail Clin. 2018;14(2):139-146. doi:10.1016/j.hfc.2017.12.004 3. Hartman JJ, Hwee DT, Robert-Paganin J, et al. Aficamten is a small-molecule cardiac myosin inhibitor designed to treat hypertrophic cardiomyopathy. Nat Cardiovasc Res. 2024;3(8):1003-1016. doi:10.1038/s44161-024-00505-0 4. Cooper GM. Actin, myosin, and cell movement. In: The Cell: A Molecular Approach. 2nd ed. Sinauer Associates; 2000. 5. Coats CJ, Masri A, Nassif ME, et al. Dosing and safety profile of aficamten in symptomatic obstructive hypertrophic cardiomyopathy: results from SEQUOIA-HCM. J Am Heart Assoc. 2024;13(15):e035993. doi:10.1161/JAHA.124.035993
