WARNING: RISK OF HEART FAILURE
MYQORZO reduces left ventricular ejection fraction (LVEF) and can cause heart failure due to systolic dysfunction.
Echocardiogram assessments are required prior to and during treatment with MYQORZO to monitor for systolic dysfunction. Initiation of MYQORZO in patients with LVEF <55% is not recommended. Decrease the dose of MYQORZO if LVEF is <50% and ≥40%. Interrupt the dose of MYQORZO if LVEF <40% or if the patient experiences heart failure symptoms or worsening clinical status due to systolic dysfunction.
Because of the risk of heart failure due to systolic dysfunction, MYQORZO is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the MYQORZO REMS Program.
CONTRAINDICATIONS
MYQORZO is contraindicated with concomitant use of rifampin.
WARNING AND PRECAUTIONS
Heart Failure
MYQORZO reduces cardiac contractility, which can reduce LVEF and cause heart failure.
Patients who experience a serious intercurrent illness (eg, serious infection) or arrhythmia (eg, new or uncontrolled atrial fibrillation) may be at greater risk of developing systolic dysfunction and heart failure.
Assess patients’ clinical status and LVEF prior to and during treatment and adjust the MYQORZO dose accordingly. New or worsening arrhythmia, dyspnea, chest pain, fatigue, leg edema, or elevations in N-terminal pro-B-type natriuretic peptide may be signs and symptoms of heart failure.
Initiation of MYQORZO in patients with LVEF <55% is not recommended.
MYQORZO REMS Program
MYQORZO is available only through a restricted program called the MYQORZO REMS Program, because of the risk of heart failure due to systolic dysfunction.
Notable requirements of the MYQORZO REMS Program include:
- Prescribers must be certified by enrolling in the MYQORZO REMS Program
- Patients must enroll in the MYQORZO REMS Program and comply with ongoing monitoring requirements
- Pharmacies must be certified by enrolling in the MYQORZO REMS Program and must only dispense to patients who are authorized to receive MYQORZO
- Wholesalers and distributors must only distribute to certified pharmacies
Further information is available at www.MYQORZOREMS.com, or at 1-844-285-7367.
Cytochrome P450 Interactions Leading to Heart Failure or Loss of Effectiveness
MYQORZO is metabolized primarily by CYP2C9, and to a lesser extent by CYP3A, CYP2D6, and CYP2C19 enzymes. Initiation of medications that inhibit multiple P450 pathways of MYQORZO elimination (eg, fluconazole, voriconazole, or fluvoxamine) or strong CYP2C9 inhibitors, and discontinuation of moderate-to-strong CYP3A inducers may lead to increased blood concentrations of aficamten and increase the risk of heart failure due to systolic dysfunction. Conversely, initiation of medications that induce P450 pathways of MYQORZO (eg, rifampin, moderate-to-strong CYP3A inducers) may lead to decreased blood concentrations of aficamten and potential loss of effectiveness. Assess LVEF 2 to 8 weeks after initiation of such inhibitors or after discontinuation of such inducers and adjust the dose of MYQORZO accordingly.
Advise patients of the potential for drug interactions. Advise patients to inform their healthcare provider of all concomitant medications prior to and during MYQORZO treatment.
ADVERSE REACTIONS
Hypertension (8% vs 2%) was the only adverse reaction occurring in >5% of patients and more commonly on MYQORZO than on placebo in the pivotal trial.
INDICATIONS AND USAGE
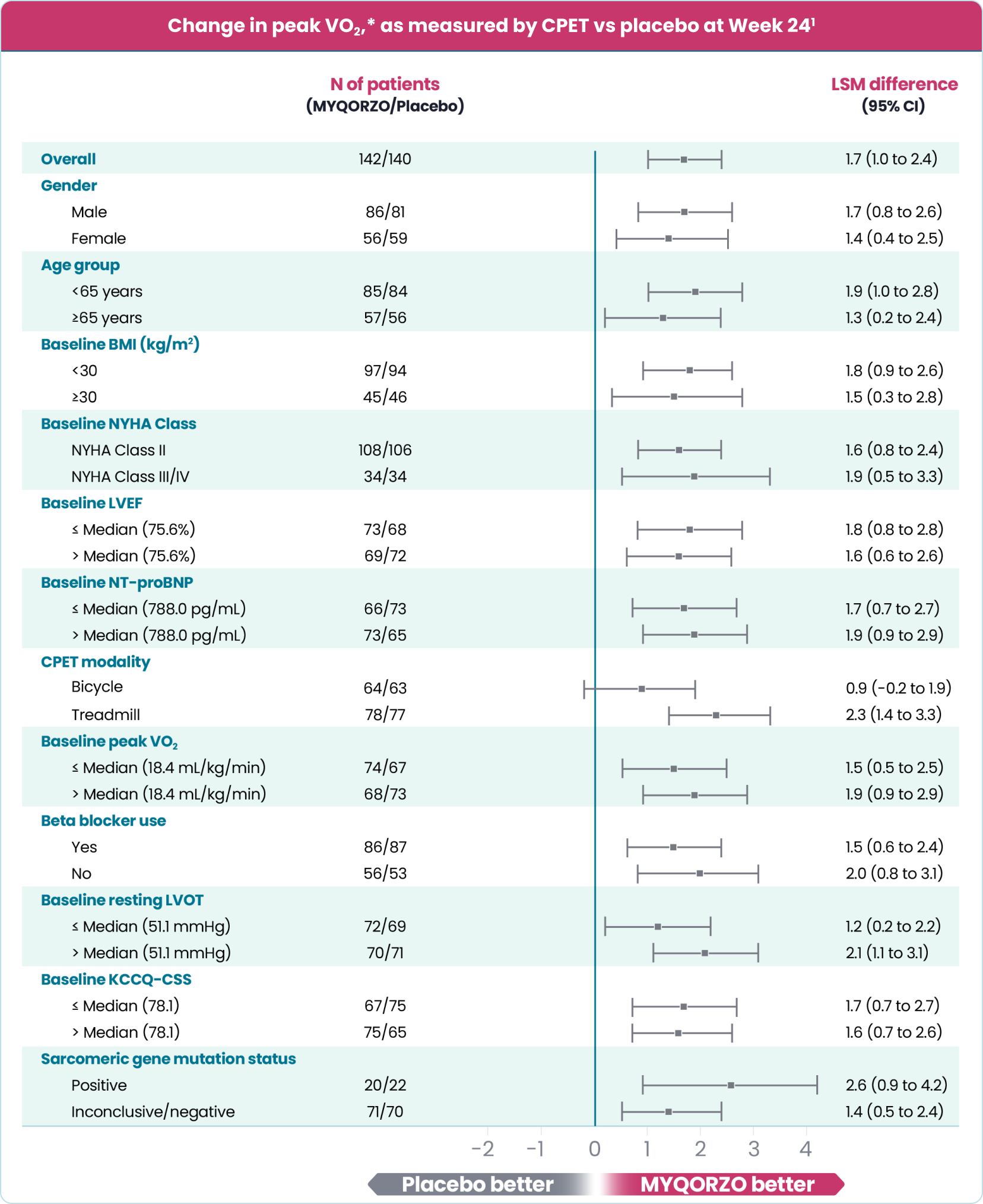
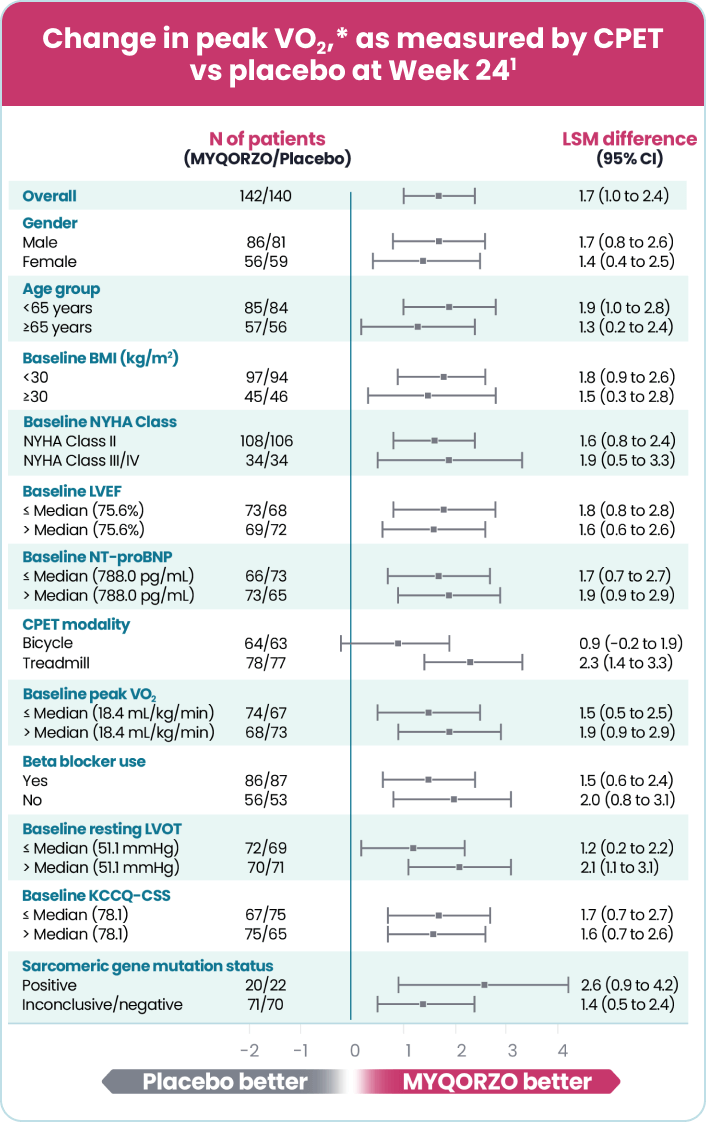
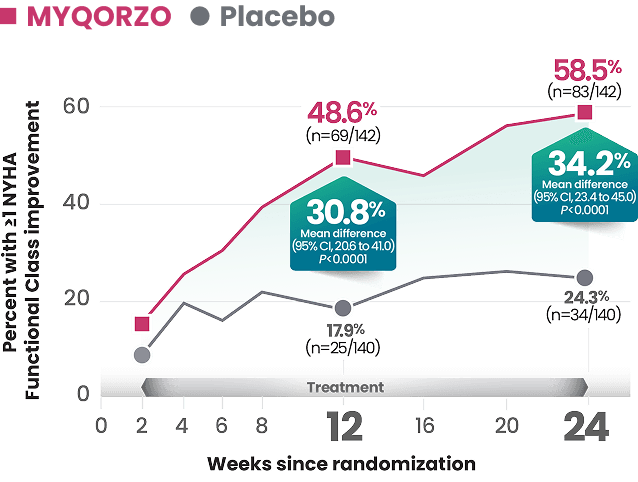
MYQORZO is indicated for the treatment of adults with symptomatic obstructive hypertrophic cardiomyopathy (oHCM) to improve functional capacity and symptoms.